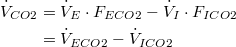
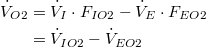
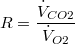
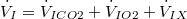
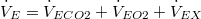
Therefore, at steady state:
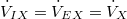
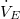
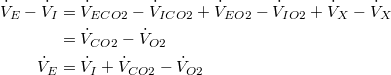
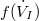
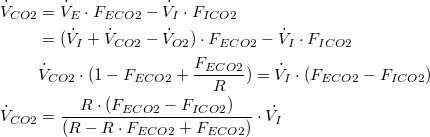
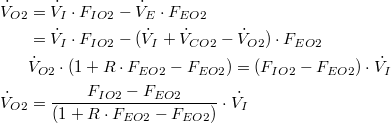
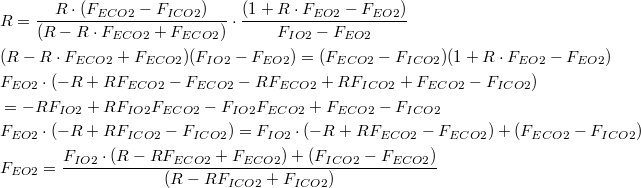
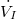
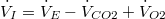
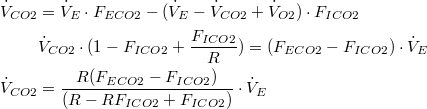
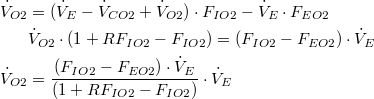
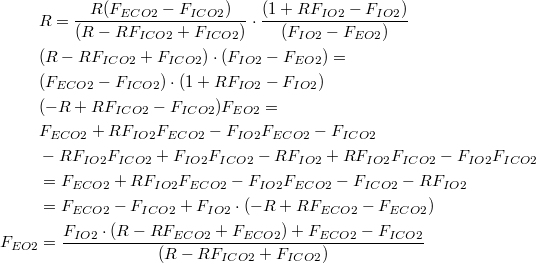
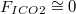
Therefore, (10) reduces to:
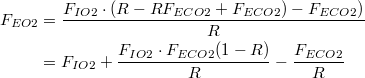
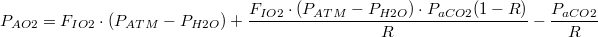
| Net CO2 production: | |
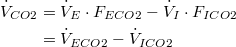 | (1) |
| Net O2 consumption: | |
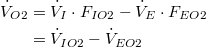 | (2) |
| Respiratory quotient: | |
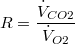 | (3) |
| Inspired & expired gas composition: | |
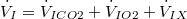 | (4) |
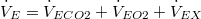 | (5) |
| X represents gases that are neither produced nor excreted. Therefore, at steady state: | |
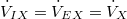 | (6) |
Solving for 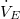 | |
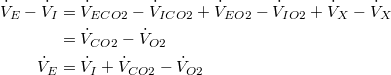 | (7) |
Using (7) to restate (1) and (2) as 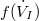 | |
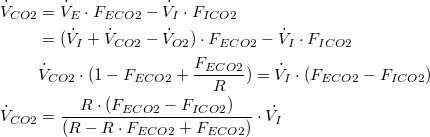 | (8) |
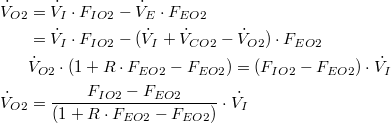 | (9) |
| Substituting (8) and (9) into (3): | |
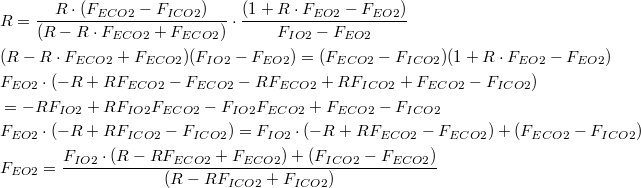 | (10) |
The same result can be obtained by solving for 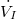 | |
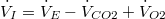 | (7a) |
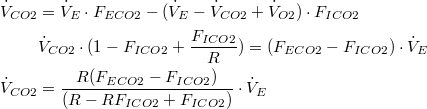 | (8a) |
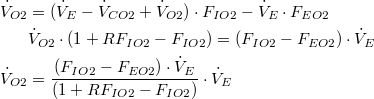 | (9a) |
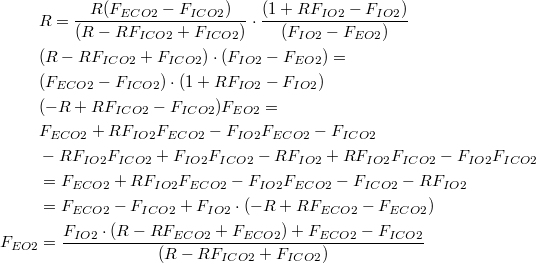 | (10a) |
Under normal physiologic circumstances, 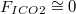 Therefore, (10) reduces to: | |
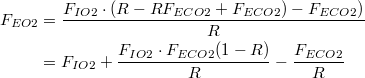 | (11) |
| By Dalton's law of partial pressures, the full humidification of alveolar air and the high diffusibility of CO2: | |
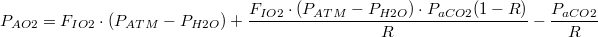 | (12) |