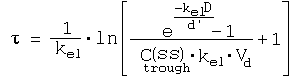
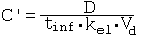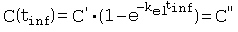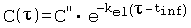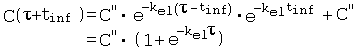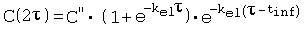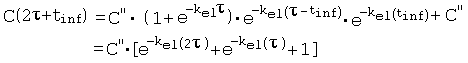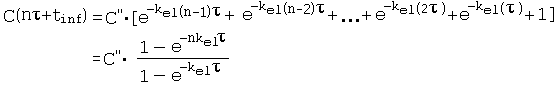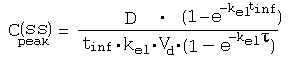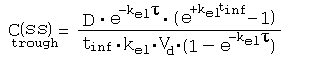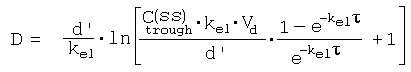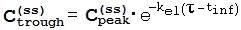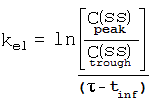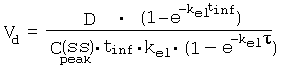References
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anethesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:3167-3184. <http://circ.ahajournals.org/cgi/reprint/111/23/e394>
- American Thoracic Society: Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. <http://ajrccm.atsjournals.org/cgi/reprint/171/4/388>
- Bauer LA. Vancomycin. In: Applied clinical pharmacokinetics. New York: McGraw Hill, Medical Publishing Division; 2001:180-261. Preview
- Birt JK, Chandler MH. Using clinical data to determine vancomycin dosing parameters. Ther Drug Monit 1990 Mar;12(2):206-9. Abstract
- Buelga DS, del Mar Fernandez de Gatta M, Herrera EV, Dominguez-Gil A, Garcia MJ. Population pharmacokinetic analysis of vancomycin in patients with hematological malignancies. Antimicrob Agents Chemother 2005 Dec;49(12):4934-41
<http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1315926> - Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16:31-41
- Columbia University Medical Center. Vancomycin Dosing and Monitoring in Adults. <http://www.cumc.columbia.edu/dept/id/downloads/Vancomycin_5-26-05.pdf>
- Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm. 1974; 8:650-5.
- Jelliffe RW. Creatinine clearance: bedside estimate [Letter to the Editor]. Ann Intern Med 1973; 79: 604-605.
- Kingston General Hospital. Vancomycin Guidelines for Dosing and Monitoring in Adults. Drug Information Bulletin 2006; 39(2): 1-4.<http://www.kgh.on.ca/pharmacy/diBulletinApr2006.pdf>
- Lee E, Winter ME, Boro MS. Comparing two predictive methods for determining serum vancomycin concentrations at a Veterans Affairs Medical Center. Am J Health Syst Pharm 2006 Oct 1;63(19):1872-5.
<http://www.ajhp.org/cgi/reprint/63/19/1872> - Leonard AE, Boro MS, Vancomycin pharmacokinetics in middle-aged and elderly men. Am J Hosp Pharm. 1994; 51:798-800. Abstract
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group Ann Intern Med 1999 Mar 16; 130 (6): 461-70.
- Levey AS, Coresh J, Greene T. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006 Aug 15;145(4):247-54.
- Levey AS, Stevens LA, Schmid C, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009 May 5;150(9):604-12. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2763564/pdf/nihms132246.pdf>
- Martin L, Chatelut E, Boneu A, Rostaing L, Roussilhes C, Caselles O, Canal P. Improvement of the Cockcroft-Gault equation for predicting glomerular filtration rate in cancer patients. Bull Cancer 1998 Jul;85(7) 631-6. Abstract
- Matzke, GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob. Agents Chemother. 1984; 25:433-437. <http://aac.asm.org/cgi/reprint/25/4/433>
- Mosteller RD: Simplified Calculation of Body Surface Area. N Engl J Med 1987 Oct 22;317(17):1098 (letter)
- Pierce, M. Vancomycin Dosing: Overview of Dosing Charts. <http://prodruginfo.com/Formulary/P&TReviews/Vancomycin_Dosing_Chart_Intranet.pdf>
- Pierce, M. Vancomycin Pharmacokinetic Dosing: Analysis of Patient Data and Optimization of Kinetic Parameters. June 2007.
<http://prodruginfo.com/Formulary/DUE/Vancomycin%20Pharmacokinetic%20Dosing%20DUE.pdf> - Rodvold KA, Blum RA, Fischer JH et al. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother 1988; 32:848-52.
<http://aac.asm.org/cgi/reprint/32/6/848> - Quetelet A. Recherches sur le poids de l'homme aux different ages. In: Nouveaux Memoire de l'Academie Royale des Sciences et Belles-Lettres de Bruxelles. (1832) t. VII.
- Rushing TA, Ambrose PJ. Clinical application and evaluation of vancomycin dosing in adults. J Pharm Technol 2001; 17:33-8. Abstract
- Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann.Intern.Med (2004) 141:929-937. <http://www.annals.org/content/141/12/929.full.pdf>
- St Luke's Hospital/Mercy Medical Center: Vancomycin Protocol. <www.crjointpt.com/library/Vancomycin%20(2.2).doc>
- Salazar DE, Corcoran GB: Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med 84: 1053-1060, 1988 Abstract
- Vance-Bryan K, Guay DRP, Gilliland SS, Rodvold KA, ROtschafer JC. Effect of Obesity on Vancomycin Pharmacokinetic Parameters as Determined by Using a Bayesian Forecasting Technique. Antimicrob. Agents Chemother. 1993; 37(3):436-440.
<http://aac.asm.org/cgi/reprint/37/3/436.pdf> - Wright JG, Boddy AV, Highley M et al. Estimation of glomerular filtration rate in cancer patients. Br J Cancer 2001; 84: 452-459.
<http://www.nature.com/bjc/journal/v84/n4/pdf/6691643a.pdf>